Antibodies: Monoclonal and Polyclonal and their Differences
In the study and treatment of infectious diseases such as Covid-19 and Human Immunodeficiency Virus or HIV, one frequently hears the term “antibodies”. Antibodies are protein elements produced by the immune system. They fortify the human body’s defence mechanism to combat bacterial, viral diseases, or antigens and obstruct them from affecting human cells.
Antibodies constitute Y-shaped immunoglobulin molecules (Ig) produced by B lymphocytes or plasma cells that activate the primary response of the adaptive immune system when a foreign molecule is detected. Because antibodies can bind antigen epitopes (the region of an antigen where an antibody binds) with great affinity and accuracy, they are essential to host immunity and are valuable in research and medicine for the treatment of disease and health improvement.
Today labs are striving to find cures and preventive treatments for diseases by developing synthetic antibody therapies which replicate and act like natural antibodies, improving the innate immune responses of the human body. Over the years, scientists have successfully developed two types of antibody therapies: polyclonal and monoclonal. Due to variations in specificity and affinity, these antibody therapies offer scientists distinctive approaches to detecting or quantifying target antigens.
In this article, medical experts from Helvetica Health Care (HHC) go into greater detail about polyclonal and monoclonal antibodies and discuss the potential uses for each kind.
In biotechnology, it can be challenging for researchers to find the appropriate antibody for their protein immunoassay. In this article, we also highlight the differences between these two antibody therapies to consider before choosing the right one.
HHC, based in Geneva, Switzerland, is a world leader in the supply of a comprehensive range of monoclonal and polyclonal antibodies against infectious agents. These antibodies are directed against specific human viral proteins and are highly specific in immunoassays.
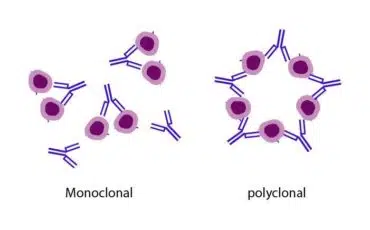
Monoclonal antibodies (MAbs):
Once an antibody is developed to detect and target specific pathogens, scientists can replicate or clone the antibody in a lab. Antibodies thus created are called monoclonal antibodies, also called monobodies.
Polyclonal antibodies (PAbs):
Polyclonal antibodies result from a combination of different antibodies produced by various B lymphocyte lineages. PAbs are a collection of IgG molecules that bind to different epitopes on a target antigen.
Difference between Monoclonal and Polyclonal antibodies
The table below summarises the main differences between the two antibody therapies and further understand their uses in medical research.
Uses of the PAbs and MAbs.
PAbs and MAbs can also be differentiated in terms of their uses in clinical research. When discussing their usefulness, we must consider their sensitivity and specificity to a pathogen. Specificity refers to how well an antibody’s binding domain binds to the target antigen in the presence of other molecules. Many applications use polyclonal and monoclonal antibodies to identify target molecules uniquely. For immunological researchers and clinicians, exploiting this factor is crucial. The sensitivity of a particular antibody is also a determining factor during experiments.
Due to their high specificity, monoclonal antibodies are used extensively for research applications that need specificity to a single epitope. Given their ability to recognise low levels of the target antigen, monospecific antibodies are equally instrumental in quantifying the levels of a particular protein.
For these reasons, monobodies are exploited for diagnostic applications such as radioimmunoassay or ELISA immunoprecipitation, immunohistochemistry, lateral flow assay, immunofluorescence assay (IF) and Western Blotting (WB). Manufacturing therapeutic antibodies is thus a promising strategy for drug development.
However, monoclonal antibodies can sometimes prove inadequate for these applications subject to the epitope as they are more prone to binding modifications when labelled. So, denatured states of the target protein structure caused by temperature, chemicals or pH can influence the efficiency of the monoclonal antibodies. In this case, the use of polyclonal antibodies might be considered.
Polyclonal antibodies have high sensitivity and strong affinity but weak specificity. Because of their strong affinity for the target antigen and tolerance to small changes in the target protein, polyclonal antibodies are helpful for many general research applications and diagnostic procedures. When polyclonal antibodies are created against a denatured antigen, they can identify the target protein’s denatured epitopes.
When performing analyses like immunoprecipitation and ELISAs, both polyclonal and monoclonal antibodies are utilised for the detection of a target protein in solution. Due to their high specificity to the target antigen, monoclonal antibodies are frequently used first (as the primary reagent) in indirect and sandwich ELISAs. Polyclonal antibodies, on the other hand, are more useful as secondary reagents because of their capacity to amplify a weak signal with high sensitivity.
When the manufacturer confirms antibody specificity and sensitivity, tests can be performed versatilely as immunoassays like ELISAs can take on different forms depending on how the antigens and antibodies are handled.
In terms of public health improvement, the immune system’s capacity to combat disease targets can be improved, imitated, and restored using antibodies. Compared to polyclonal antibodies, monoclonal antibodies are a better option for therapeutic purposes as they have a high specificity to a single epitope, a lower probability of cross-reactivity and are homogenous.
They are highly effective for cancer therapies. Monoclonal antibody therapies work against cancer cells that can evade or block the immune system by direct binding to induce cell death. They can also block tumours from growth factors and blood supply.
Recombinant monoclonal antibodies
Recombinant antibodies are the latest technology in the production of monoclonal antibody therapies. Over the last two decades, the production of recombinant monoclonal antibodies for therapeutic applications has grown exponentially. Scientists have developed numerous recombinant monoclonal antibodies to target and fight against infectious diseases and human viral proteins such as Ebola and Covid-19 that represent increased potential health risks.
We at HHC believe that health can be improved with efficient medical research and high-quality lab tests and applications. Based in Geneva, Switzerland, HHC can provide you with a vast range of MONOBODIES™ that are highly specific in immunoassays and can be used in different applications, including ELISA, immunoblotting and immunochemistry and produce therapies that can fight against infectious agents.
We deliver our products in a temperature-sensitive, quick, and efficient way. Contact us if you want to know we can help improve your lab testing efficiency and performance.
